The collection of micro-organisms and virus on culture media requires time for incubation before results. Instead, much faster results are obtained applying the liquid collection method.
This method has great advantages when applied in the following fields:
- Industrial segment (pharma, agro-food, beverage, etc.) for a quick reaction in identifying the contamination
- Military branches for notifying possible biological attacks
- Hospitals for finding the correct pharmacological product and treatment for patients
- Public institutes (school, restaurant, bar, metro, train, municipality buildings, etc.) for disease/pandemic monitoring
AIRBIO RAPID-VIRUS also allows a quick assessment of the disinfection protocols' efficacy.
The instrument is the result of the European NATO project EUCLID CEPA 13 (“protection of personnel against pathogenic micro-organisms via air sampling and rapid detection and identification”).
Principle: the volume of air is aspirated and mixed in a pre-analytical liquid.
- The collection liquid system is completely sterilizable as produced in stainless steel
- Collection liquid: water, buffer, nutrient broth
- Quantity of collection liquid: 15 ml
- Battery autonomy: 70.000 litres
- 50 users and 50 places
- Dimension of the instrument: 15x20x33h cm
- Weight: 1.850 gr
- Battery: power supply - 218 VDC 60W
- Operating conditions: T° 0-45°C / RH 10% / 60%
| Code |
AIRBIO ONE RAPID-VIRUS |
| 2448K |
AIRBIO ONE RAPID-VIRUS PACK consisting of: AIRBIO ONE air sampler 200 l/m, 1 s/s Petri aspirating head with s/s cover head, 1 s/s collection liquid system for virus, 2 conical tube PP (225 ml), battery charger, 1 calibration certificate and 1 robustus carrying case. |
| Code |
Accessories |
| 324 |
Conical tube PP - 225 ml (8 x box) |
| 413 |
Self-sealing sterilization bag for s/s collection liquid system for AIRBIO RAPID-VIRUS (100 x box) |
| 325 |
Collecting liquid system by PCR for AIRBIO VIRUS |
| 329 |
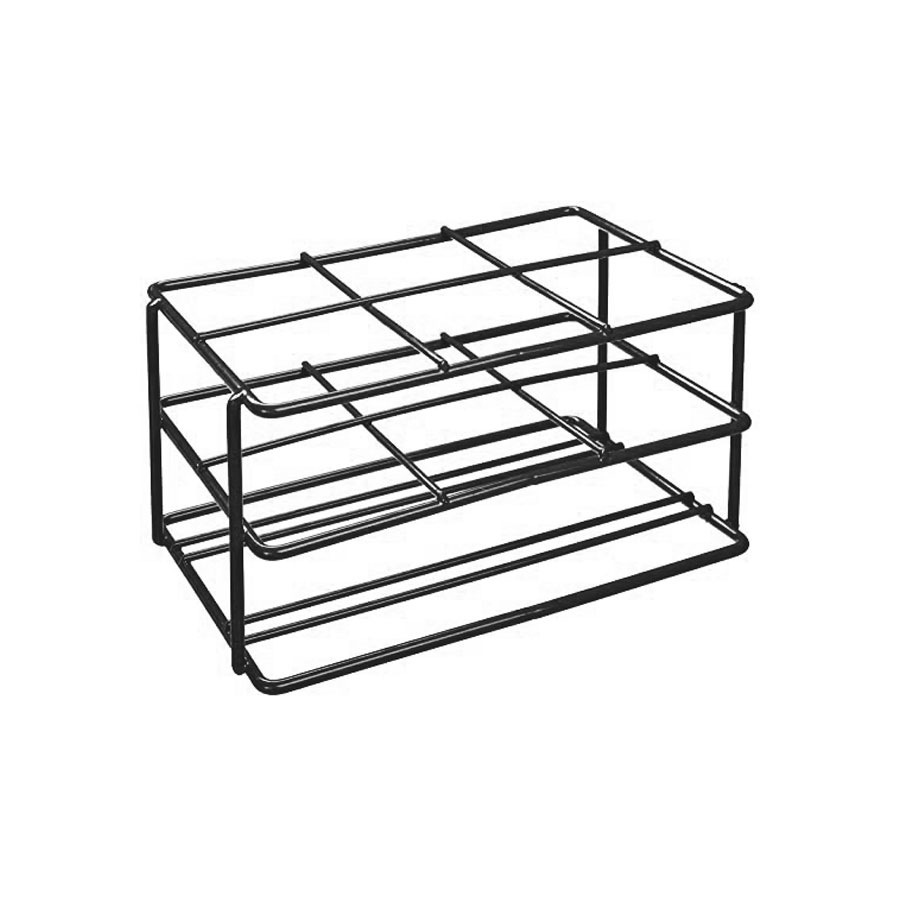
VIRUS rack for conical tube PP - size 205x135x102 mm |
| Sampling air volumes |
The suggested volume of air is 2.000 liters.
The air sampler has an autonomy of 70.000 litres, but it could be more whist connected to the main power. |
| Sampling place |
The air sampler should be positioned on the direction of the air flow (between door and windows, close to the air conditioning system of HVAC). In hospital, the sampler should be placed near to patient’s bed, in public spaces where there is the highest concentration of people. |
| Sampling Protocol |
A specific SOP (Standard Operative Procedure) needs to be prepared. |
| Collection liquid |
The most common used liquid is phosphate buffer or saline buffer. The volume should be 15 ml. In case the aspirating time is longer, it should be necessary to add sterile water to PBS to avoid salt concentration. |
| Sample Storage |
If the analytical sample is not processed right away, the sample needs to be stored at +4°C. |
| Sample transfer |
The sample should be transferred at +4°C unless different indications from analytical laboratory. |
| Sample processing |
Some protocols indicate a concentration’s step (by tangential flow filtration). |

Sampling data management Software
The evolution of microbial environmental monitoring in cleanroom “from paper to paperless”
Until the sampling data remains on the air sampler, it is not easy for the operator to check it and keep a record while the sampling inventory is full. A customized software, uploaded on a PC, allows the customer to view the data in an easier format, to print it for further analysis or controls by inspectors.
The software allows the customer to move from a paper world to a paperless one.
There are two different software available: the AS Software for a simple data transfer and the BAS SW which is Data integrity compliant. The BAS SW is specifically dedicated to the pharma field.
Read more
Airbone Virus Monitoring in Public Indoor Settings and High Risk Hospital Wards
INTRODUCTION
The transmission of virus in indoor environments
It is recognised by the Centre for Disease Control and Prevention (CDC) and World Health Organisation (WHO) that aerosol transmission in poorly ventilated indoor environments may play a significant role in viral exposure.
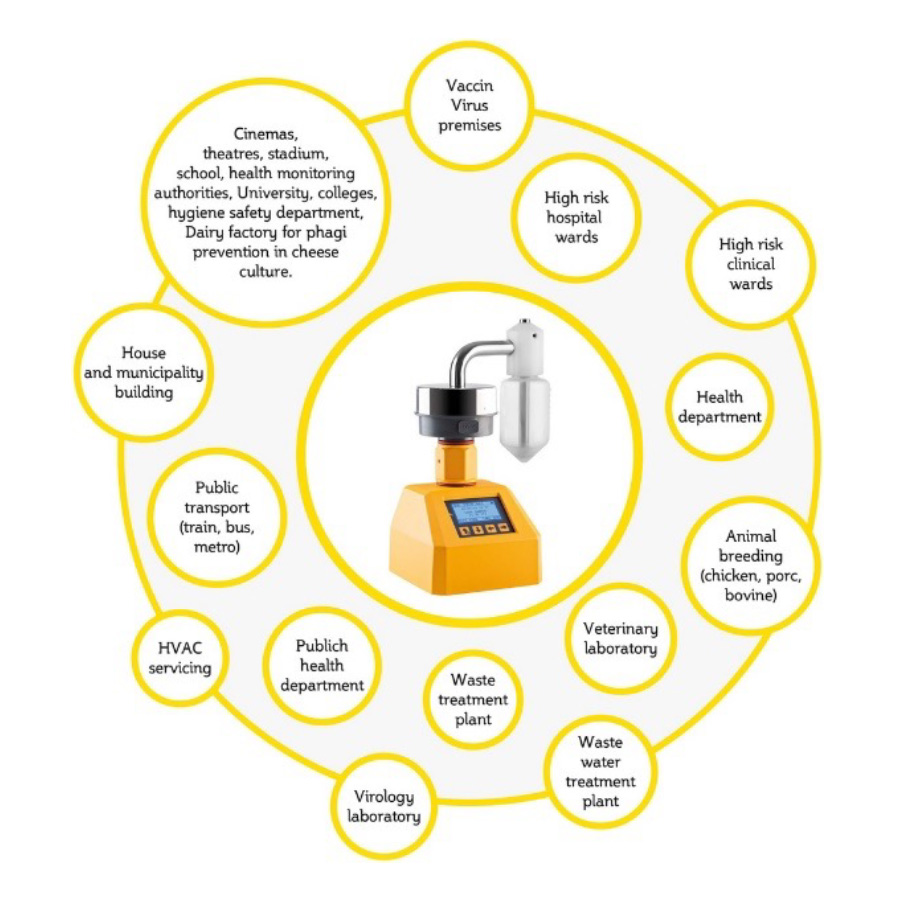
Hospital, Clinic, Industry should monitor for the presence of potential pathogens in the air with the purpose to mitigate the risk of infections to protect staff and patients.
Air monitoring is especially important if possible contamination by SARS-COV – viral particles is expected.
VIRUSES AND AEROSOL
The spreading of viruses
The effects of indoor viral exposure during a pandemic are a concern for industries such as hospitals, health clinics, air travel, hospitality, theatres, convention centres and venues receiving large public populations.
Small droplets remain in the air longer and travel farther than large droplets. If aerosol contain viruses in sufficient quantity, a susceptible person could inhale them and become infected.
The monitoring of indoor air environments by quantitative analysis is an important tool to provide data for risk assessment to evaluate the viral exposure.
The new approach to collect viruses from air
The traditional test to collect respiratory viruses from air requested a filtration with subsequent culture and long time to reach the result. The new approach with a liquid samples obtained by the AIRBIO.ONE RAPID VIRUS instrument and rapid PCR type techniques can give results in a few hours.
The AIRBIO.ONE RAPID VIRUS is specifically designed for viruses but also for total pathogen surveillance of bacteria, fungi, yeast.
The instrument consists of a command unit connected to a conical sampling device that holds a collecting liquid. The environmental air is aspirated into the collecting liquid and this allows for capture of viral particles. The liquid sample is subsequently submitted for testing by rapid analytical test methods such as PCR or microarrays.
The origin of the AIRBIO.ONE RAPID VIRUS
The instrument is the result of the European NATO project EUCLID CEPA 13 “Protection of personnel against pathogenic micro-organisms via air sampling and rapid detection and identification”.
The sampling strategy and sample analysis protocol for viruses
Several parameters should be considered when designing a sampling strategy. One important point is the positioning of the sampler. It should be placed in the trajectory of the airflow of the place.
In case of hospital ward, it should be placed at about 100 cm from the bed of patient.
It is responsibility of the analytical laboratory the type and volume of liquid to be used, the amount of air to be collected (e.g.: 2.000 litres) and how to preserve the nucleic acids of the sample.
A positive control is suggested during the DNA/RNA extraction protocol.
The practical use of the AIRBIO.ONE RAPID VIRUS
- Take out the protective cover from AIRBIO Command unit
- Connect the sterilised collection device to the aspirating chamber of AIRBIO Command unit
- Disconnect the protective bottle of the collection device
- Connect the bottle containing the sterile collection liquid
- The Air sampler is ready to be used
- Press the on switch of the Command unit
- At the end of aspirating cycle, take out the bottle, apply the cap and transfer it to the laboratory.
- The sample is ready to be analysed.
The sterilisation of the AIRBIO.ONE RAPID VIRUS
The system can be sterilised on the field using sterile 70% alcohol or by autoclaving at 120°C for 20 minutes.
The method and the liquid to be used for the molecular tests
It is the task of the molecular laboratory to indicate the collecting liquid and the rapid test to be adopted (e.g.: qPCR, microarray, NGS.).
The temperature to transfer the liquid sample to the laboratory.
The sample should be at +4°C (unless a different indication is received by the lab).
AIRBIO.ONE RAPID VIRUS for pathogen collection
The sampler can also be used for bacteria, fungi, yeast with the liquid protocol or with traditional culture Petri dishes.
Application of the AIRBIO.ONE RAPID VIRUS
CONCLUSIONS
The AIRBIO.ONE RAPID VIRUS aerosol sampler is in ideal instrument to be used in combination with the rapid molecular test like PCR to obtain the results in short time or traditional conventional Petri agar culture based tests.
- AIR SAMPLING STRATEGY: place, time, frequency according to validated SOP
- AIR SAMPLER PREPARATION: volume of air and type of liquid according to the analytical laboratory
- AIR SAMPLER PROGRAMMING: air sampler protocol according to user manual and validated SOP
- SAMPLES COLLECTION: after using a sterile conical tube, place a new sterile tube on the collection liquid system for a new test
- SAMPLES: PCR test, qPCR RT, qPCR
- AIR SAMPLER DECONTAMINATION: use 70% isopropyl alcohol
- NEW SAMPLE: the air sampler is ready for a new test

 English
English
 Arabic
Arabic
 Chinese simplified
Chinese simplified
 Dutch
Dutch
 French
French
 German
German
 Japanese
Japanese
 Korean
Korean
 Portuguese
Portuguese
 Russian
Russian
 Spanish
Spanish
 Ukrainian
Ukrainian

 SEARCH
SEARCH